| |
| Anticorrosion |
| |
| Principle of Anticorrosion Performance of BIO-POWER |
Main causes of heat erosion
Organics or inorganic metals and sulfide contained in fuel oil will form fine droplets during the course of combustion. Those fine droplets will flow with the air and stick on the furnace body or metal surface, thus a kind of compound with low melting point and high corrosivity is generated, which may corrode the furnace body and machine members. |
| The following conditions will result in heat erosion: |
| |
| Na2SO4 high temperature sulfuration erosion ( MP. 884℃) |
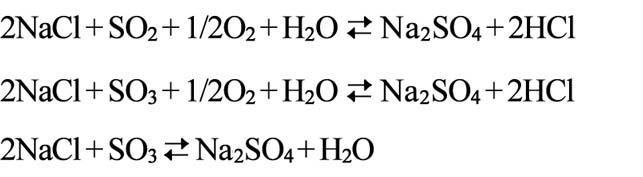 |
| When partial pressure of Na2SO4 is greater than equilibrium pressure, Na2SO4 will begin to get coagulated and stick on metal surface, and make chemical reactions with oxides such as Cr2O3 and form Na2O•Cr2O3 is a kind of low melting point compound, it will destroy protective layer. The sulfur generated from chemical reaction will penetrate into metal interior of and cause sulfidation, and thus protection of oxide layer of Cr2O3 is reduced. In addition, Na2SO4 will make direct chemical reaction with V2O5 and form V2O5•Na2SO4 is a kind of low melting point compound salt, which deteriorates erosion. |
| |
V2O5 Vanadium Attack (MP. 690℃)
When in low temperature, vanadium will take presence in the form of V2O3 or V2O4, which is relatively stable and of low volatility. When high temperature is reached, carbide contained in fuel oil will be completely used up, and V2O5 (MP.690℃)will generate under this situation. Some vanadium will combine sodium, nickel, iron, magnesium, calcium etc. (melting point 500℃ ~ 1200℃) and form vanadium compound directly. While some vanadium will condense into fusion state and form low melting point compounds such as Cr2O3•V2O5 (MP.665℃), NiO•V2O5 (MP.640℃) with Cr2O3 and NiO contained in the protective layer on metal surface. Those compounds will destroy the protective layer and finally, deteriorate erosion. |
| |
| High temperature caused by PbO metal oxide |
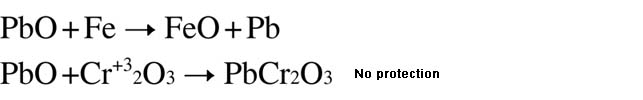 |
| PbO、MoO3、V2O5 are of prominent corrosivity to steel materials. Although Bi2O3 (MP. 824℃), B2O3 (MP. 460℃) and Sb2O3 (MP. 656℃) belong to low melting point metal oxides, they have no corrosivity even in liquid state after fusion. When pure iron, which is cover by mixed liquids such as Na2O、B2O3、Na2O•GeO2, is pre-heating treated for 2 hours under high temperature of 1200℃, no acceleration of oxidation will take place. Therefore, low melting point metal oxides in addition to diffusion velocity and electronic conductivity will accelerate high temperature erosion. |
| |
Fine solid state granules in heavy oil
Fine semi-melt solid state matters will generate after oxidation of combustion, they will attack the furnace body and thus abrasion is formed. |
|
|
 |



